Quality by design

It is not a new concept and in fact the father figure on Quality, Juran, in his book, wrote about quality by design as far back as 1992. He wrote about principles used to advance product and process quality in the automotive industry.
These principles have been borrowed by the Pharma Industry. The ICH has, in its Guidelines Q8 and Q8(R), incorporated these principles as technical requirements for registration of Pharmaceuticals for human use.
The ICH as all should know, stands for International Council on Harmonisation started off as a conference between 3 regions to harmonise requirements for submission for new drug products. The 3 regions were USA, EU and Japan, the 3 major and most stringent nations for Pharma registrations.
Now ICH has grown and is accepted as a bible for regulations. The founding Regulatory Members are EC – Europe, FDA – US, and MHLW/PMDA – Japan. There are 2 bodies who are standing observers IFPMA and WHO. The ICH Guidelines are divided into 4 topics or categories. The Q series pertain to the area of Quality. All Guidelines in this are prefixed with Q followed by a number.
So what is QbD for Pharma? The ICH Q8(R) describes it as “A systematic approach to development that begins with predefined objectives and emphasises product and process understanding and process control, based on sound science and quality risk management”.
What does this mean exactly?
Pharmaceutical development i.e. when new products are developed the process traditionally has been a model of trying different processes and testing the product. This can be either very cumbersome or wholly dependent on the immediate test results without any idea of what any manufacturing or raw material variations would do to the product during its life to expiry.
The desired process is one by which quality is built in right from the start. How is this done? Firstly identify, understand, and control CQA’s (Critical Quality Attributes) and CPP’s (Critical Process Parameters). Develop a Design Space (DS) to identify acceptable limits of operation via DOE (Design of Experiments). Institute a control strategy to ensure that production (i.e. process) is maintained within the DS.
So how does one develop a DS?
In ICH Q8 (R2), DS is defined as “the multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality.” Working within this space is not considered as a change and hence does not require regulatory approval. Design space can be described in terms of ranges of material attributes and process parameters, or through more complex mathematical relationships. It is generally determined through statistically designed experiment such as Design of Experiment (DoE). This enables maximum information with minimum experimental trials. Design space is only for CPP or critical material attributes that has direct impact to product CQA. It can be established for each unit operation or for a span of a few unit operations or even for the entire process.
So if you are developing a new solid dose form you could establish the DS for mixing, dry/wet blending, granulating, compressing etc. Once you have the multi-dimensional combination and interaction of input variables and process parameters that have been demonstrated to provide assurance of quality you have established your Design Space. Schematically it would be something like this:
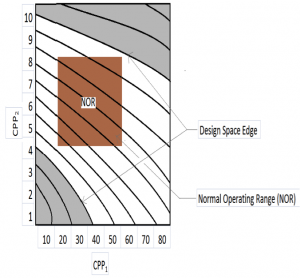
What are the advantages of doing new product development as given above?
Better innovation due to the ability to improve processes without resubmission to the FDA when remaining in the Design Space. Greater regulatory confidence of robust products. Less batch failures when manufactured within the DS. Improved yields, less investigations, reduced testing and lower costs. Also more efficient technology transfer i.e. transfer of process from lab to production floor or from one facility to another.
Very often during technology transfer the material and process may remain the same but the equipment may be different. This is where QbD and working within the design space becomes important. What does one do when equipment used in development is not available at commercial scale? Selecting a manufacturing unit with similar mixing styles to what was used in the development program is the next best way forward. An awareness of where the product will be manufactured commercially helps the development team design a process suitable for the intended contract manufacturer. None of these challenges are insurmountable provided the development scientists, formulators, and engineers have done a thorough job of characterising the product and process and have established the design space. In other words built in the quality by design.
Categories
Recent Posts
Subscribe
Never miss a post from Fabtech. Sign up to receive updates direct to your inbox.
