Hot Melt Extrusion Process in Pharmaceutical Drug Formulation

Formulators rely on science and technology knowledge for solutions to new or existing problems through well-considered combinations of methodologies and approaches that profoundly impact the healthcare and pharmaceutical industry. They understand and evaluate the pre-formulation phase, formulation and process development, and post-formulation phases to achieve specific objectives. Hot Melt Extrusion, a prevalent practice in the plastic industry, is now widely practised and holds a prominent significance in modern drug formulation development in the pharmaceutical industry.
HME Principle:
The drug or polymeric materials are processed continuously above their glass transition temperature and pressure in a controlled manner to achieve molecular level mixing of thermoplastic binders and/or polymers and active compounds (drugs).
HME’s Working Process:
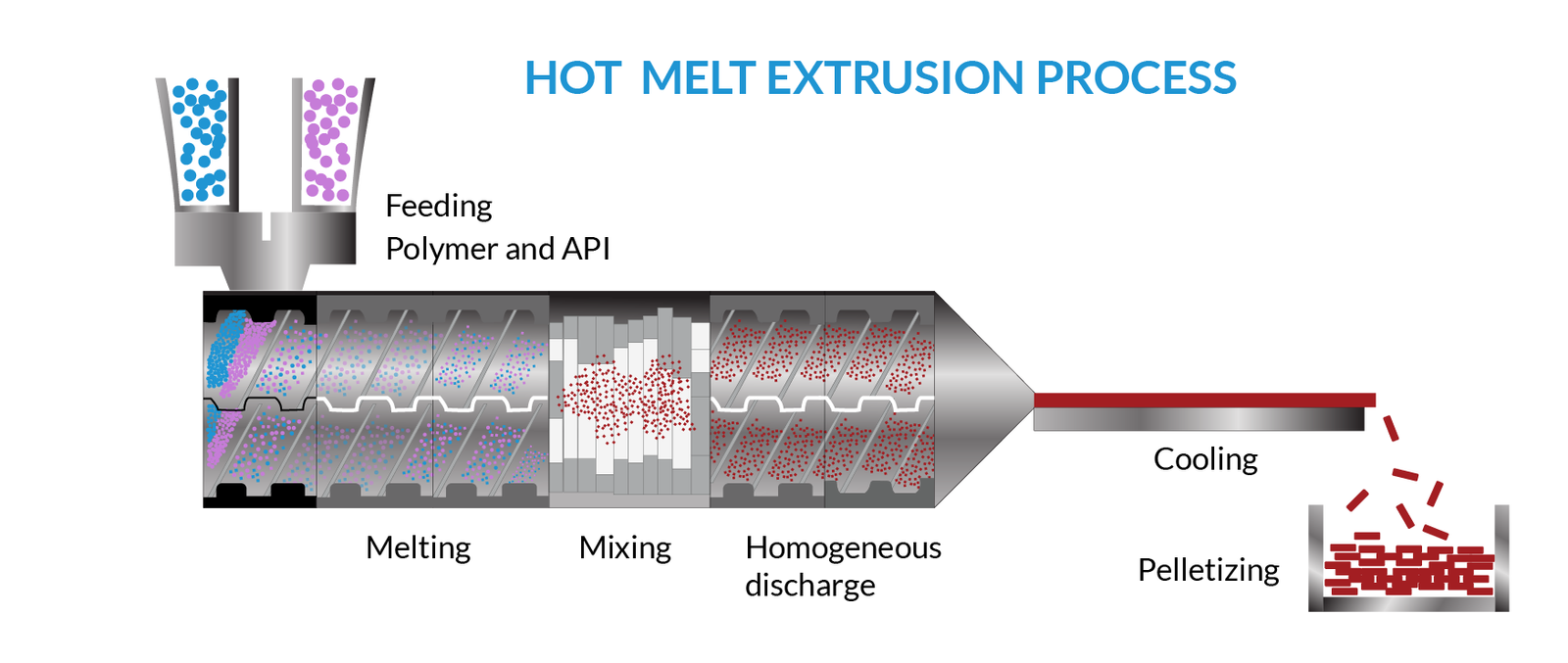
The process of mixing a drug with one or more excipients like diluents, plasticisers, or polymers through heat and pressure treatment causes the material to melt. Moreover, pushing it through an orifice in a continuous process helps produce uniform products shape and density for industrial applications.
The Hot Melt Extrusion process is optimised with an electronic control unit in a screw extruder, allowing it to set screw speed, process temperature, and pressure. Heat and shear force or stress causes the Screw extruder to generate a homogenous blend that impacts the final product characteristics.
The two types of screws within the extruder i.e.single-screw and twin-screw extruders, for different mixes as the material, move through the barrel. The single-screw extrusion system has a single rotating screw inside the barrel and further separated into feed, compression and metering zones that causes a shift in pressure within each of these zones that produces a mix of the excipients and API. The single screw extrusion’s uninterrupted movement creates high pressure and is especially suitable for highly viscous material.
In the twin-screw extruder, two screws are arranged generally side by side, and each screw can rotate in the same or the opposite direction. The twin-screw extruder offers greater versatility due to the design arrangement and a high degree of mixing as the mass’s residence time increases. This can process a wider range of pharmaceutical formulations.
The length diameter ratio (L/D) of both single and twin-extruders is a vital aspect that affects the final product’s characteristics. However, the ease of consolidating different batch operations into a single continuous process contributing to an increase in manufacturing efficiency makes twin-screw extrusion popular.
Design and Optimisation
The design and optimisation of the HME process involve various factors.
- Process factors: Appropriate equipment, mass feed rate, process temperature, shear stresses.
- Physiochemical factors: Drug and excipient properties, Physical and chemical compatibility between the mix and stability components
- Formulation factors: Drug-polymer interactions, drug-polymer solubility, Rheological properties, Physical state and drug dissolution from extrudes.
- Process Analytical Technologies (PAT) with Design of Experiments results in successfully optimising the HME process.
Dosage form and routes of administration:
Dosage forms such as granules, pellets, implants can be prepared using the HME process. These dosage forms can be later converted to tablets, capsules, and granules for suspension and administered through oral, transmucosal and intradermal delivery.
Advantages:
- Absence of solvents: Reduces the risk of chemical degradation due to hydrolysis while using aqueous-based solvents and reduces toxicity related risk while using organic solvents.
- Improved Bio-availability: Increasing solubility leads to increasing the bio-availability of poorly soluble drugs.
- Modified drug release: Can produce granules for delayed, sustained or extended-release formulation by mixing drug and polymer at specific solubility.
- Drug content Uniformity: Uniform distribution of drugs in the malted mass improves drug uniformity in individual units’ final formulation.
- Process Scalability: It’s a continuous operation and easily scalable with process analytical technology.
Disadvantages
- Due to the thermal process, considering drug-polymer stability, only limited polymers can be used.
- The process needs high flow properties of polymers and excipients.
- Not suitable for relatively high heat-sensitive molecules such as microbial species and proteins
Case Studies:
Many proven studies on drug molecules are processed through the Hot Melt Extrusion process to improve the drug dissolution profile and increase drug bioavailability. This may be either in the form of granules, pellets or spheres — the expected drug releases either in the form of immediate or modified release from such dosage forms. Here are a few examples – Diltiazem Hydrochloride, Theophylline, Ibuprofen, Carbamazepine, Itraconazole, Ketoconazole, Ketoprofen etc.
However, these drugs may include matrix carriers, release modifying agents, bulking agents, antioxidants, thermal lubricants, and miscellaneous additives when processed along with the functional excipients. The excipients listed under these categories maintain their properties. Hence a compatibility study of these excipients with drugs is to be necessary before formulating the recipe.
Conclusion:
The Hot Melt Extrusion process offers versatility and flexibility to the production process, providing an excellent alternative to conventional processes like spray drying. The choice of raw materials, extruder accessories (affects the final feeding equipment), screw design, post extrusion equipment help set the right process for different dosage forms.
Categories
Recent Posts
Subscribe
Never miss a post from Fabtech. Sign up to receive updates direct to your inbox.
